A covalent bond can best be described as. Dwhich has the maximum number of carbon-hydrogen bonds possible.
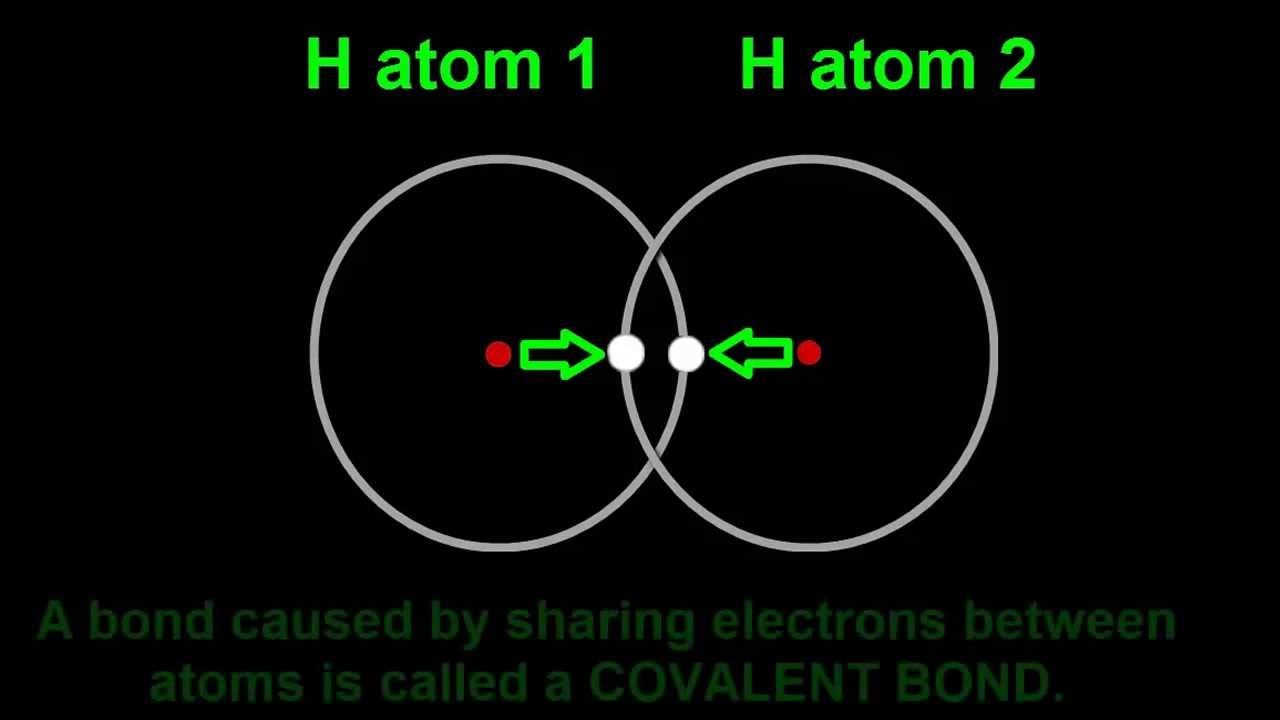
How Two Hydrogen Atoms Join To Become A Hydrogen Molecule H2 Covalent Bonding Molecules Hydrogen Atom
Dissolving is best described as a change from a solid to a liquid.

. 12 11 2p 1 1s 2s boron carbon silicon nitrogen fluorine D. How many pairs of electrons are shared between the two nitrogen atoms. A have a branching carbon skeleton B have different combinations of double bonds between carbon atoms C have different positions of double bonds between carbon atoms D form enantiomers D 17 The two molecules shown in the figure below are best described as _____.
Carbon has _____ potential bonding sites. Hydrogen bonding requires that a hydrogen atom is bonded to carbon. Which of the following best describes an isotope.
A 10-year bond with a 5 coupon and a 1million VND par value is currently priced at 821000VND. This hydrogen atom can then form a hydrogen bond with an O N or F on another molecule. Isocyanic acid 615 methanamine 293 Describe in terms of electrons the type of bonding between the carbon atom and the nitrogen atom in a molecule of methanamine.
An inorganic molecule may have one of these but not both. Organic molecules contain at least A three carbon to hydrogen bonds. Cwhich is formed from many smaller molecules.
A Nonpolar covalent B Polar covalent C Ionic D Coordinate covalent E None of these Ans. Up to 24 cash back Awhich contains one or more multiple bonds between carbon atoms. Molecules breaking into ions.
What is dissolving best described as. Which bond is best described as an intermolecular attraction due to partial charges formed in polar covalent bonds. Which of the following is responsible for the cohesive property of water.
Examples include glycogen and starch. A mingling of molecules andor ions. 1 2The process used to produce simple.
B one carbon to oxygen bond. Methanol CH3OH which can be used as a fuel can be formed by the reaction between carbon monoxide and hydrogen as depicted in the following balanced equation. Covalent bonds involve the sharing of electrons between atoms.
The bond between carbon and hydrogen is best described as O A. The carbon hydrogen bond is covalent in nature. Induction and Polar Covalent bond Section.
Carbohydrates are a large and diverse group of molecules composed of carbon hydrogen and oxygen. Compound Formula Carbon-Nitrogen Bond Energy kJmol hydrogen cyanide 890. Hydrogen bonding requires that a hydrogen atom is bonded to O N or F.
State the relationship between the number of electrons in a carbon-nitrogen bond and carbon-nitrogen. Athe carbon valence electrons only Bthe hydrogen valence electrons only Cthe carbon and hydrogen valence electrons. Carbon shares its outer valence electrons with upto 4 hydrogens Atoms make bonds to become stableAs hydrogen carries 1 electron and it need 1 more electron to be stable or to complete its duplet in the same way carbon needs 4 more electrons to complete its octate so by sharing the outer 4 valence.
Distinguish between organic and inorganic molecules. Polar covalent O C. A covalent bond is formed between a carbon atom and a nitrogen atom along with the formation of H2O.
Which best describes the intermolecular force known as hydrogen bonding. A covalent bond is formed between a carbon atom and a nitrogen atom along with the formation of H2OH2O. E one hydrogen to oxygen bond.
A covalent bond is formed between a carbon atom and a nitrogen atom along with the formation ofH2OH2O. Bwhich can react by taking up one or more water molecules. An organic molecule has a carbon backbone and at least one carbon to hydrogen bond.
An atomic structural variation in which atoms have differing numbers of neutrons. D one carbon to hydrogen bond. C one ionic bond.
Ewith a specific six-membered ring structure. Ionic bonds involve the electrical attraction between charged atoms. 2H2 CO CH3OH Suppose 356 g of CO and 65 g of H2 are mixed.
The bond between carbon and hydrogen is best described as_____. Nonpolar covalent O B. Bonds between carbon and hydrogen atoms as depicted on the right side of the figure are generally _____ nonpolar covalent bonds.
4As energy is released during the formation of a bond the stability of the chemical system generally will A1 B2 C3 D4 5Given the formula for hydrazine. Hydrogen bonds between the oxygen atom of one water molecule and. Which element has the following electronic configuration.

Chemistry Covalent Bonding Lesson Activities Covalent Bonding Chemistry Lessons Lessons Activities

Structure Hydrocarbon A Compound Composed Only Of Carbon And Hydrogen Chemistry Organic Chemistry Molecules

Four Covalent Bonds Carbon Has Four Valence Electrons And Here A Valence Of Four Each Hydrogen Atom Has One Vale Covalent Bonding Chemical Bond Ionic Bonding
0 Comments